Cutting-Edge Research
Advancing Neuroscience Solutions
The Rana Lab in the Department of Neuroscience is committed to cutting-edge research, integrity, collaboration, and a relentless pursuit of scientific excellence in studying neural control of breathing in disease and injury.
Adam Caar
Developer
Use this space to introduce yourself and share your professional history.
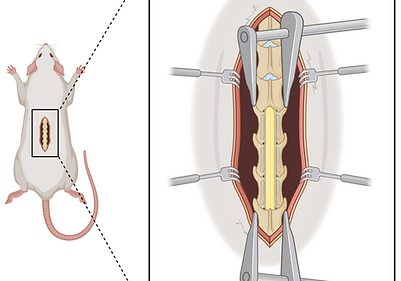
Spinal cord injury
Models
We have extensive expertise in experimental models of spinal cord injury in both rats and mice, spanning clinically relevant contusion and crush paradigms to reductionist hemisection and complete transection approaches designed for mechanistic hypothesis testing. Injuries are implemented at defined cervical and thoracic levels to selectively interrogate distinct sensorimotor and autonomic domains, including respiratory control, bladder function, cardiovascular and autonomic regulation, and forelimb or hindlimb motor function. This multi model, level specific strategy enables rigorous investigation of circuit level pathophysiology while preserving strong translational relevance to human spinal cord injury.
Adam Caar
Developer
Use this space to introduce yourself and share your professional history.
In vivo respiratory assessments


The Rana Lab integrates complementary physiological and neurophysiological approaches to quantify respiratory motor function across behavioral states and injury conditions. These methods enable high resolution, longitudinal assessment of breathing and respiratory circuit output in both awake and anesthetized preparations. Core techniques include:
Whole body plethysmography (DSI) in awake animals to measure ventilatory patterning, respiratory frequency, tidal volume, and responses to physiological challenges in a noninvasive, behaviorally relevant context.
Indwelling electromyography (EMG) electrode implantation to record diaphragm and accessory respiratory muscle activity in awake and anesthetized animals using a longitudinal, repeated measures design that enables within subject tracking across injury and recovery.
Peripheral nerve recordings from phrenic and hypoglossal nerves to directly quantify respiratory motor output to pump and upper airway muscles, providing mechanistic insight into neural circuit function and plasticity.
Adam Caar
Developer
Use this space to introduce yourself and share your professional history.

Neuromodulation
We are developing noninvasive spinal stimulation strategies to modulate neural circuit excitability and improve motor function after neurological injury and disease. Our work integrates transcutaneous spinal stimulation approaches (direct current stimulation) with quantitative physiological and neurophysiological readouts to define stimulation parameters, state dependent effects, and mechanisms of action across respiratory and somatic motor systems. These methods are implemented within controlled experimental and longitudinal study designs to evaluate therapeutic efficacy, durability of response, and interactions with complementary pharmacological or activity based interventions.
Neuropharmacology

The Rana Lab evaluates targeted neuropharmacological strategies (ampakines, trk receptor mimetic peptides) designed to enhance motor system excitability and restore functional output in states of neurological disease and spinal cord injury. We focus on agents that modulate synaptic transmission, neuromodulatory tone, and activity dependent plasticity within defined motor circuits. These interventions are rigorously paired with quantifiable neurophysiological outcomes, to directly assess changes in motor unit recruitment, firing patterns, and circuit level responsiveness.

Gene Therapy
The Rana Lab employs gene therapy approaches to selectively target spinal motor pools and modulate defined neural circuits involved in motor control. A major and expanding focus of our work is the development and application of optogenetic and chemogenetic tools that enable precise, cell specific manipulation of respiratory motor circuits across physiological and injury contexts. These strategies are integrated with quantitative neurophysiology to determine how circuit organization, excitability, and plasticity shape functional motor output. Together, this platform supports mechanistic discovery while informing the design of next generation, circuit targeted therapeutic approaches for neurological disease and spinal cord injury.